The influence of an acid catalyst on the morphology, wettabillity, adhesion and chemical structure properties of TiO2 and ZrO2 sol-gel thin films
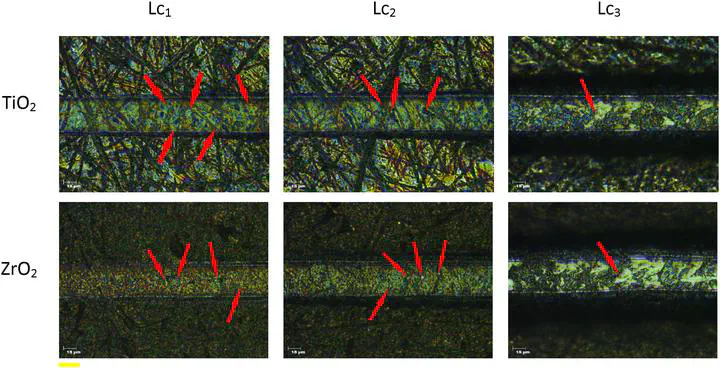 Failures characteristic for critical normal forces (Lc1, Lc2, Lc3) for TiO2 and ZrO2 coatings obtained with use of HNO3 as catalyst (red arrows indicate the specific failures).
Failures characteristic for critical normal forces (Lc1, Lc2, Lc3) for TiO2 and ZrO2 coatings obtained with use of HNO3 as catalyst (red arrows indicate the specific failures).Streszczenie
The proper selection of synthesis parameters and the way of preparing materials is of crucial importance in successful sol–gel synthesis, hence high quality of coatings is essential. The pH value of the sol–gel reaction mixture, which is dependent on the used catalyst, influences the hydrolysis and condensation reaction and also affects the form and structure of the received materials. Acids contribute to protonate alkoxide groups and intensify the hydrolysis reaction kinetics. Moreover, during condensation the pH affects the structure of the produced polymeric chains. Acid-catalyzed reaction is preferentially directed to the end of chains and leads to the achievement of less branched and more extended structures. The studies presented in this paper, focused on the influence of a catalyst type used in the acid catalysis for the preparation of thin films. TiO2 and ZrO2 thin films have been investigated due to the ability to form crystalline structure at relatively low temperatures. The influence of the used acid catalyst (HCl, HNO3, H2SO4, H3PO4) on physicochemical properties was considered. SEM examination of the obtained materials was carried out to check the influence of different catalysts on the surface morphology and topography. The impact of the type of the used catalyst on the chemical structure of material has been investigated by Raman and Infrared Spectroscopy. Wettability measurements were conducted to verify the catalyst and materials structure effect on surface properties of the obtained materials. All described results have proved that sol–gel process of zirconia materials is much more sensitive to the pH value of a reaction in comparison with titania materials.